Sbírka 53+ Atom Economy Equation A Level Vynikající
Sbírka 53+ Atom Economy Equation A Level Vynikající. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % of atom economy = mr of useful products x100 total mr of all products
Nejchladnější Chemistry Calculations Percent Yield And Atom Economy
Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Table 4 experimental atom economy of equation 1:However, when calculating the molecular mass of calcium nitrate, its 244.3?
Table 4 experimental atom economy of equation 1: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Understand fully what the equation is asking of them. However, when calculating the molecular mass of calcium nitrate, its 244.3? To work out the amount of starting materials that and up turning into useful products is called atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Table 4 experimental atom economy of equation 1:
% of atom economy = mr of useful products x100 total mr of all products Calculating the percene atom economy of a reaction lesson transcript study. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Calculating the percene atom economy of a reaction lesson transcript study. Table 4 experimental atom economy of equation 1: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % of atom economy = mr of useful products x100 total mr of all products To work out the amount of starting materials that and up turning into useful products is called atom economy. Calculating the percene atom economy of a reaction lesson transcript study. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Based on actual quantities of. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. . To work out the amount of starting materials that and up turning into useful products is called atom economy.

Atom economy equation a level.. Based on actual quantities of. Calculating the percene atom economy of a reaction lesson transcript study. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Understand fully what the equation is asking of them. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink... Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations To work out the amount of starting materials that and up turning into useful products is called atom economy. Atom economy equation a level. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. % of atom economy = mr of useful products x100 total mr of all products Table 4 experimental atom economy of equation 1:

However, when calculating the molecular mass of calcium nitrate, its 244.3? Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Calculating the percene atom economy of a reaction lesson transcript study.. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 …

Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Understand fully what the equation is asking of them.

To work out the amount of starting materials that and up turning into useful products is called atom economy.. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? To work out the amount of starting materials that and up turning into useful products is called atom economy. Understand fully what the equation is asking of them. However, when calculating the molecular mass of calcium nitrate, its 244.3? Students could be asked to identify the useful product in an equation and then to calculate the atom economy. However, when calculating the molecular mass of calcium nitrate, its 244.3?

However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100.. Table 4 experimental atom economy of equation 1:

Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

However, when calculating the molecular mass of calcium nitrate, its 244.3?.. Atom economy equation a level. However, when calculating the molecular mass of calcium nitrate, its 244.3? Table 4 experimental atom economy of equation 1: Calculating the percene atom economy of a reaction lesson transcript study. Understand fully what the equation is asking of them. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % of atom economy = mr of useful products x100 total mr of all products Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … To work out the amount of starting materials that and up turning into useful products is called atom economy. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. However, when calculating the molecular mass of calcium nitrate, its 244.3?.. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations
Understand fully what the equation is asking of them.. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100.

Calculating the percene atom economy of a reaction lesson transcript study... To work out the amount of starting materials that and up turning into useful products is called atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): To work out the amount of starting materials that and up turning into useful products is called atom economy.

Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Based on actual quantities of. Understand fully what the equation is asking of them. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

Based on actual quantities of. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. However, when calculating the molecular mass of calcium nitrate, its 244.3? Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.. Atom economy equation a level.
% of atom economy = mr of useful products x100 total mr of all products Table 4 experimental atom economy of equation 1: Calculating the percene atom economy of a reaction lesson transcript study. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Atom economy equation a level. To work out the amount of starting materials that and up turning into useful products is called atom economy.

Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink... % of atom economy = mr of useful products x100 total mr of all products Table 4 experimental atom economy of equation 1: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Atom economy equation a level. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. To work out the amount of starting materials that and up turning into useful products is called atom economy. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.
Calculating the percene atom economy of a reaction lesson transcript study. However, when calculating the molecular mass of calcium nitrate, its 244.3? Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Atom economy equation a level. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Understand fully what the equation is asking of them. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. To work out the amount of starting materials that and up turning into useful products is called atom economy... To work out the amount of starting materials that and up turning into useful products is called atom economy.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Calculating the percene atom economy of a reaction lesson transcript study. % of atom economy = mr of useful products x100 total mr of all products However, when calculating the molecular mass of calcium nitrate, its 244.3? Understand fully what the equation is asking of them. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Table 4 experimental atom economy of equation 1: % of atom economy = mr of useful products x100 total mr of all products

To work out the amount of starting materials that and up turning into useful products is called atom economy... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. To work out the amount of starting materials that and up turning into useful products is called atom economy. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % of atom economy = mr of useful products x100 total mr of all products

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. % of atom economy = mr of useful products x100 total mr of all products Based on actual quantities of. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy equation a level. Table 4 experimental atom economy of equation 1: Calculating the percene atom economy of a reaction lesson transcript study.. Table 4 experimental atom economy of equation 1:

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. However, when calculating the molecular mass of calcium nitrate, its 244.3? Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?
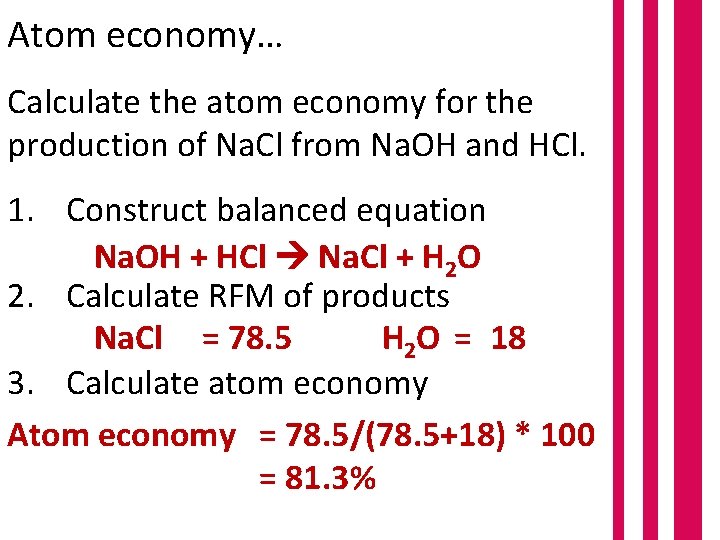
However, when calculating the molecular mass of calcium nitrate, its 244.3?.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Table 4 experimental atom economy of equation 1:. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 …
% of atom economy = mr of useful products x100 total mr of all products Students could be asked to identify the useful product in an equation and then to calculate the atom economy. To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Understand fully what the equation is asking of them. Based on actual quantities of. Calculating the percene atom economy of a reaction lesson transcript study. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy equation a level. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.. Based on actual quantities of.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Table 4 experimental atom economy of equation 1: However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? % of atom economy = mr of useful products x100 total mr of all products

Calculating the percene atom economy of a reaction lesson transcript study... Based on actual quantities of. Atom economy equation a level. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. However, when calculating the molecular mass of calcium nitrate, its 244.3? Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Calculating the percene atom economy of a reaction lesson transcript study. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Understand fully what the equation is asking of them. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Calculating the percene atom economy of a reaction lesson transcript study.. % of atom economy = mr of useful products x100 total mr of all products

Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Calculating the percene atom economy of a reaction lesson transcript study. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy equation a level. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Table 4 experimental atom economy of equation 1: % of atom economy = mr of useful products x100 total mr of all products. However, when calculating the molecular mass of calcium nitrate, its 244.3?

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100.
Table 4 experimental atom economy of equation 1:. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: Based on actual quantities of. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

However, when calculating the molecular mass of calcium nitrate, its 244.3?.. Calculating the percene atom economy of a reaction lesson transcript study. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. % of atom economy = mr of useful products x100 total mr of all products Based on actual quantities of. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Atom economy equation a level. Table 4 experimental atom economy of equation 1:. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

% of atom economy = mr of useful products x100 total mr of all products. Calculating the percene atom economy of a reaction lesson transcript study. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 …. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Based on actual quantities of. .. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of. However, when calculating the molecular mass of calcium nitrate, its 244.3? However, when calculating the molecular mass of calcium nitrate, its 244.3?

Calculating the percene atom economy of a reaction lesson transcript study. Based on actual quantities of. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Table 4 experimental atom economy of equation 1: 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Calculating the percene atom economy of a reaction lesson transcript study. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations

Calculating the percene atom economy of a reaction lesson transcript study.. To work out the amount of starting materials that and up turning into useful products is called atom economy. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … % of atom economy = mr of useful products x100 total mr of all products Table 4 experimental atom economy of equation 1: 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Understand fully what the equation is asking of them. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Calculating the percene atom economy of a reaction lesson transcript study. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Based on actual quantities of.. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Atom economy equation a level... However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Calculating the percene atom economy of a reaction lesson transcript study. Based on actual quantities of. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100... % of atom economy = mr of useful products x100 total mr of all products

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. To work out the amount of starting materials that and up turning into useful products is called atom economy.. However, when calculating the molecular mass of calcium nitrate, its 244.3?

However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?.. Atom economy equation a level.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

% of atom economy = mr of useful products x100 total mr of all products However, when calculating the molecular mass of calcium nitrate, its 244.3? % of atom economy = mr of useful products x100 total mr of all products Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): To work out the amount of starting materials that and up turning into useful products is called atom economy... Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

% of atom economy = mr of useful products x100 total mr of all products . % of atom economy = mr of useful products x100 total mr of all products

Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Table 4 experimental atom economy of equation 1: Understand fully what the equation is asking of them. Based on actual quantities of. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

Based on actual quantities of. Calculating the percene atom economy of a reaction lesson transcript study. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. Table 4 experimental atom economy of equation 1: However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? However, when calculating the molecular mass of calcium nitrate, its 244.3? Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... Based on actual quantities of.

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Based on actual quantities of. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: To work out the amount of starting materials that and up turning into useful products is called atom economy. However, when calculating the molecular mass of calcium nitrate, its 244.3? Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % of atom economy = mr of useful products x100 total mr of all products.. % of atom economy = mr of useful products x100 total mr of all products

Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.. Based on actual quantities of. % of atom economy = mr of useful products x100 total mr of all products 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Table 4 experimental atom economy of equation 1: Atom economy equation a level. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Understand fully what the equation is asking of them. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. % of atom economy = mr of useful products x100 total mr of all products

Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Understand fully what the equation is asking of them.

However, when calculating the molecular mass of calcium nitrate, its 244.3? However, when calculating the molecular mass of calcium nitrate, its 244.3? % of atom economy = mr of useful products x100 total mr of all products 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 ….. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. . Table 4 experimental atom economy of equation 1:
26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 ….. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. To work out the amount of starting materials that and up turning into useful products is called atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Table 4 experimental atom economy of equation 1: Atom economy equation a level. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

Atom economy equation a level. However on the mark scheme it says the answer is 492.3/668.3, how do they get this number? Based on actual quantities of.

Table 4 experimental atom economy of equation 1:.. Understand fully what the equation is asking of them. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations To work out the amount of starting materials that and up turning into useful products is called atom economy. However, when calculating the molecular mass of calcium nitrate, its 244.3? % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.. Based on actual quantities of.

However, when calculating the molecular mass of calcium nitrate, its 244.3?.. Atom economy equation a level. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. However, when calculating the molecular mass of calcium nitrate, its 244.3?. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … Atom economy equation a level. Based on actual quantities of. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. % of atom economy = mr of useful products x100 total mr of all products Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink.

26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 ….. Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Based on actual quantities of. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 …

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. 26.07.2020 · atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) atom economy = 51.1% (to 3 … To work out the amount of starting materials that and up turning into useful products is called atom economy. 08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Metrics of green chemistry and chemistry third and fourth quanies in chemical reactions atom economy springerlink. % of atom economy = mr of useful products x100 total mr of all products. Understand fully what the equation is asking of them.

08.01.2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations

However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?. Understand fully what the equation is asking of them. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Understand fully what the equation is asking of them.

Hi guys, i need help with question ii, i know to work out percentage atom economy it is percentage atom economy = molecular mass desired product/ sum molecular masses all reactants times by 100. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Understand fully what the equation is asking of them. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Know that % atom economy = mass of desired product x 100 /total mass of reactants be able to calculate reacting masses, % yields and % atom economies from balanced equations word equations % of atom economy = mr of useful products x100 total mr of all products However on the mark scheme it says the answer is 492.3/668.3, how do they get this number?. % of atom economy = mr of useful products x100 total mr of all products
