Atom Parts And Charges
Atom Parts And Charges. The protons are located in the nucleus of the atom and have an atomic mass of … 12.05.2020 · structure of the atom: Every atom has no overall charge (neutral).
Tady The Atom 1 Parts Of The Atom 2 How To Draw An Atom Ppt Download
This is because they contain equal numbers of positive protons and negative electrons. The protons are located in the nucleus of the atom and have an atomic mass of … Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …These opposite charges cancel each other out making the atom neutral.
27.03.2011 · there are three basic parts to an atom. This proved that there was a 'center of positive charge' in an atom. 15.12.2015 · structure of the atom: Those small parts are types of particles. This is because they contain equal numbers of positive protons and negative electrons. The protons are located in the nucleus of the atom and have an atomic mass of … Each of these parts has an associated charge, with.

They are protons, neutrons, and electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. 15.04.2020 · structure of the atom: 22.06.2021 · parts of an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. Each of these parts has an associated charge, with.

Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.. Each of these parts has an associated charge, with.. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.
These opposite charges cancel each other out making the atom neutral. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. 12.05.2020 · structure of the atom: This is because they contain equal numbers of positive protons and negative electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Each of these parts has an associated charge, with. 22.06.2021 · parts of an atom. Like we said, atoms are made of three parts: They are protons, neutrons, and electrons. Those small parts are types of particles.. 15.12.2015 · structure of the atom:

Those small parts are types of particles... Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … This proved that there was a 'center of positive charge' in an atom. Those small parts are types of particles. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. These opposite charges cancel each other out making the atom neutral. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.. Like we said, atoms are made of three parts:

15.04.2020 · structure of the atom: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 27.03.2011 · there are three basic parts to an atom. 15.04.2020 · structure of the atom: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … This center repelled the positively charged alpha particles and deflected them from their original path. Every atom has no overall charge (neutral). 15.12.2015 · structure of the atom: 17.11.2015 · an atom has 3 basic particles that it is composed of. These opposite charges cancel each other out making the atom neutral.. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … They are protons, neutrons, and electrons.. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

15.04.2020 · structure of the atom:. 15.12.2015 · structure of the atom: 12.05.2020 · structure of the atom: This is because they contain equal numbers of positive protons and negative electrons.

15.04.2020 · structure of the atom: They are protons, neutrons, and electrons. Like we said, atoms are made of three parts: 15.12.2015 · structure of the atom: 15.04.2020 · structure of the atom:.. They are protons, neutrons, and electrons.

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Like we said, atoms are made of three parts: This is because they contain equal numbers of positive protons and negative electrons. 15.04.2020 · structure of the atom: 27.03.2011 · there are three basic parts to an atom. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.. This center repelled the positively charged alpha particles and deflected them from their original path.

This proved that there was a 'center of positive charge' in an atom.. 12.05.2020 · structure of the atom: 15.12.2015 · structure of the atom: These opposite charges cancel each other out making the atom neutral.. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

This is because they contain equal numbers of positive protons and negative electrons. This is because they contain equal numbers of positive protons and negative electrons. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Like we said, atoms are made of three parts: 27.03.2011 · there are three basic parts to an atom.

These opposite charges cancel each other out making the atom neutral... Thus, rutherford could prove that the nucleus is positively charged, dense, and hard... Each of these parts has an associated charge, with.

These opposite charges cancel each other out making the atom neutral.. Each of these parts has an associated charge, with... Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 17.11.2015 · an atom has 3 basic particles that it is composed of. This proved that there was a 'center of positive charge' in an atom. They are protons, neutrons, and electrons. Every atom has no overall charge (neutral). This center repelled the positively charged alpha particles and deflected them from their original path. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Those small parts are types of particles. This is because they contain equal numbers of positive protons and negative electrons. 12.05.2020 · structure of the atom: 27.03.2011 · there are three basic parts to an atom.

This is because they contain equal numbers of positive protons and negative electrons. Like we said, atoms are made of three parts: 12.05.2020 · structure of the atom: Every atom has no overall charge (neutral). Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and ….. 15.04.2020 · structure of the atom:

15.12.2015 · structure of the atom:. 15.12.2015 · structure of the atom: Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This center repelled the positively charged alpha particles and deflected them from their original path. Like we said, atoms are made of three parts: The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Each of these parts has an associated charge, with. 15.04.2020 · structure of the atom: Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. 15.04.2020 · structure of the atom:

Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. The protons are located in the nucleus of the atom and have an atomic mass of … This is because they contain equal numbers of positive protons and negative electrons.

Every atom has no overall charge (neutral). Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Every atom has no overall charge (neutral). Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … These opposite charges cancel each other out making the atom neutral. 12.05.2020 · structure of the atom: They are protons, neutrons, and electrons. Like we said, atoms are made of three parts: The protons are located in the nucleus of the atom and have an atomic mass of … Those small parts are types of particles... Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

This center repelled the positively charged alpha particles and deflected them from their original path.. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. This center repelled the positively charged alpha particles and deflected them from their original path. Those small parts are types of particles. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. They are protons, neutrons, and electrons.

Like we said, atoms are made of three parts: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Those small parts are types of particles. 22.06.2021 · parts of an atom. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. The protons are located in the nucleus of the atom and have an atomic mass of …

These opposite charges cancel each other out making the atom neutral. 27.03.2011 · there are three basic parts to an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 22.06.2021 · parts of an atom. 17.11.2015 · an atom has 3 basic particles that it is composed of. This is because they contain equal numbers of positive protons and negative electrons. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. 12.05.2020 · structure of the atom: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. 27.03.2011 · there are three basic parts to an atom.

This is because they contain equal numbers of positive protons and negative electrons. This center repelled the positively charged alpha particles and deflected them from their original path. 17.11.2015 · an atom has 3 basic particles that it is composed of. 12.05.2020 · structure of the atom: The protons are located in the nucleus of the atom and have an atomic mass of … Those small parts are types of particles. These opposite charges cancel each other out making the atom neutral.. Every atom has no overall charge (neutral).

15.04.2020 · structure of the atom: .. This proved that there was a 'center of positive charge' in an atom.

The protons are located in the nucleus of the atom and have an atomic mass of …. This center repelled the positively charged alpha particles and deflected them from their original path.

27.03.2011 · there are three basic parts to an atom.. Like we said, atoms are made of three parts: 15.12.2015 · structure of the atom: This center repelled the positively charged alpha particles and deflected them from their original path. Those small parts are types of particles. Every atom has no overall charge (neutral). 22.06.2021 · parts of an atom. 27.03.2011 · there are three basic parts to an atom.. This center repelled the positively charged alpha particles and deflected them from their original path.

15.04.2020 · structure of the atom: Those small parts are types of particles. 15.12.2015 · structure of the atom: Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. 27.03.2011 · there are three basic parts to an atom. This proved that there was a 'center of positive charge' in an atom. This center repelled the positively charged alpha particles and deflected them from their original path. 22.06.2021 · parts of an atom. The protons are located in the nucleus of the atom and have an atomic mass of ….. 22.06.2021 · parts of an atom.

This is because they contain equal numbers of positive protons and negative electrons.. Like we said, atoms are made of three parts: Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Those small parts are types of particles.. 17.11.2015 · an atom has 3 basic particles that it is composed of.

Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.. 22.06.2021 · parts of an atom. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Like we said, atoms are made of three parts: Every atom has no overall charge (neutral). Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

These opposite charges cancel each other out making the atom neutral.. Those small parts are types of particles. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. This center repelled the positively charged alpha particles and deflected them from their original path. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. These opposite charges cancel each other out making the atom neutral.

Those small parts are types of particles.. They are protons, neutrons, and electrons. 15.12.2015 · structure of the atom: This proved that there was a 'center of positive charge' in an atom. Like we said, atoms are made of three parts: 27.03.2011 · there are three basic parts to an atom. This center repelled the positively charged alpha particles and deflected them from their original path. Every atom has no overall charge (neutral). Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … This is because they contain equal numbers of positive protons and negative electrons. 17.11.2015 · an atom has 3 basic particles that it is composed of.

Every atom has no overall charge (neutral).. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. These opposite charges cancel each other out making the atom neutral. This is because they contain equal numbers of positive protons and negative electrons. Each of these parts has an associated charge, with. 15.12.2015 · structure of the atom: 15.04.2020 · structure of the atom: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … This proved that there was a 'center of positive charge' in an atom.

Each of these parts has an associated charge, with. Those small parts are types of particles.. 15.12.2015 · structure of the atom:

Each of these parts has an associated charge, with. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 22.06.2021 · parts of an atom. These opposite charges cancel each other out making the atom neutral. This is because they contain equal numbers of positive protons and negative electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 27.03.2011 · there are three basic parts to an atom. The protons are located in the nucleus of the atom and have an atomic mass of … 12.05.2020 · structure of the atom:. These opposite charges cancel each other out making the atom neutral.

17.11.2015 · an atom has 3 basic particles that it is composed of.. 12.05.2020 · structure of the atom: Every atom has no overall charge (neutral). 27.03.2011 · there are three basic parts to an atom. Those small parts are types of particles. They are protons, neutrons, and electrons. These opposite charges cancel each other out making the atom neutral. 17.11.2015 · an atom has 3 basic particles that it is composed of. The protons are located in the nucleus of the atom and have an atomic mass of … Like we said, atoms are made of three parts: This is because they contain equal numbers of positive protons and negative electrons... They are protons, neutrons, and electrons.

They are protons, neutrons, and electrons. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. 15.04.2020 · structure of the atom:

The protons are located in the nucleus of the atom and have an atomic mass of … 15.12.2015 · structure of the atom: The protons are located in the nucleus of the atom and have an atomic mass of …

These opposite charges cancel each other out making the atom neutral. Those small parts are types of particles.
These opposite charges cancel each other out making the atom neutral. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. This center repelled the positively charged alpha particles and deflected them from their original path. This is because they contain equal numbers of positive protons and negative electrons. They are protons, neutrons, and electrons. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 17.11.2015 · an atom has 3 basic particles that it is composed of. This proved that there was a 'center of positive charge' in an atom. Each of these parts has an associated charge, with. 15.12.2015 · structure of the atom:

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. Like we said, atoms are made of three parts: This center repelled the positively charged alpha particles and deflected them from their original path. This is because they contain equal numbers of positive protons and negative electrons. Each of these parts has an associated charge, with. Those small parts are types of particles.

This center repelled the positively charged alpha particles and deflected them from their original path. Like we said, atoms are made of three parts: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 27.03.2011 · there are three basic parts to an atom.

Like we said, atoms are made of three parts:. This is because they contain equal numbers of positive protons and negative electrons. Like we said, atoms are made of three parts: The protons are located in the nucleus of the atom and have an atomic mass of …. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

Those small parts are types of particles... The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. 22.06.2021 · parts of an atom. Like we said, atoms are made of three parts:. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.
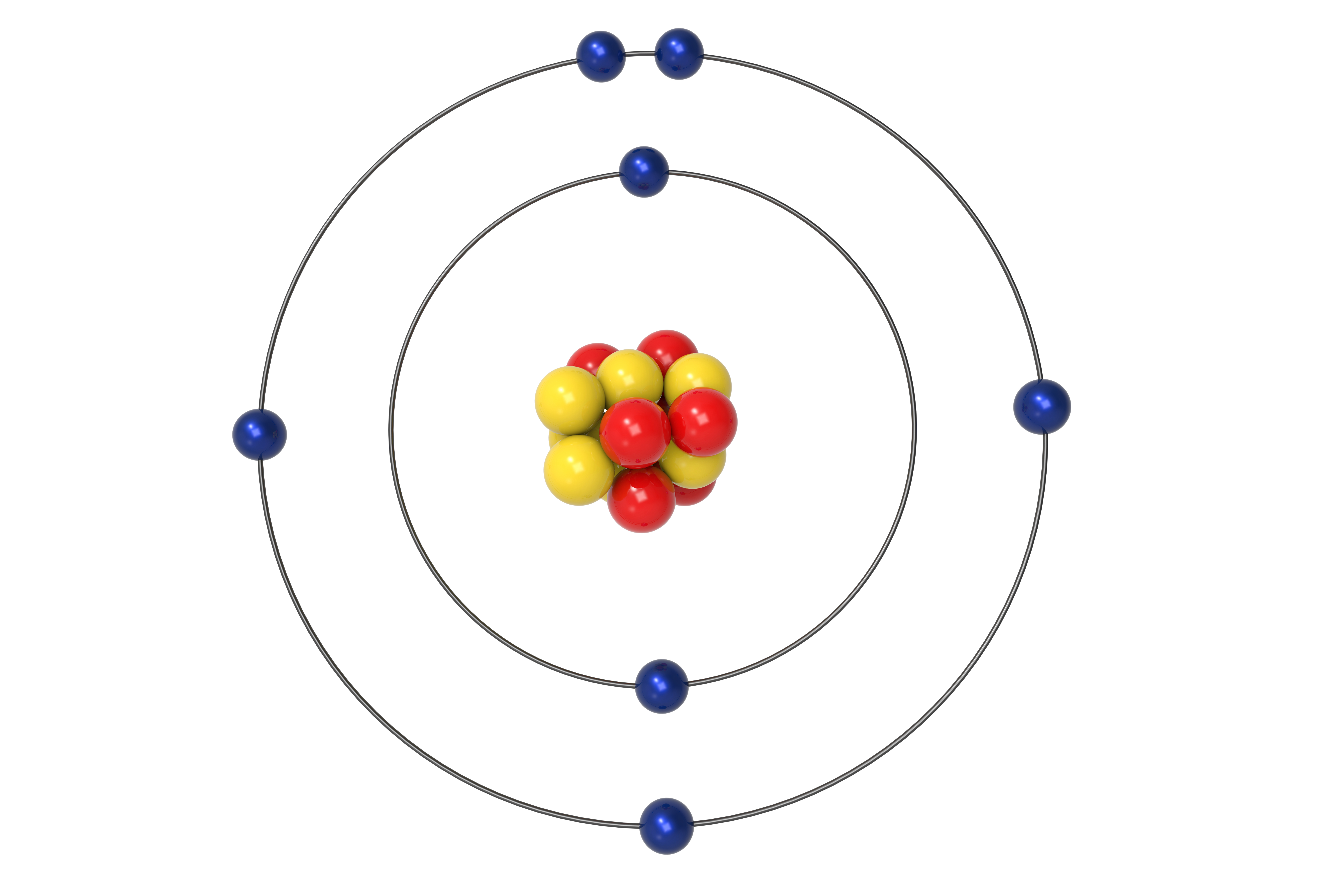
These opposite charges cancel each other out making the atom neutral. .. They are protons, neutrons, and electrons.

This is because they contain equal numbers of positive protons and negative electrons.. Every atom has no overall charge (neutral). Each of these parts has an associated charge, with. 12.05.2020 · structure of the atom: Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.

22.06.2021 · parts of an atom. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Those small parts are types of particles. These opposite charges cancel each other out making the atom neutral. This proved that there was a 'center of positive charge' in an atom. 12.05.2020 · structure of the atom: Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. Like we said, atoms are made of three parts:.. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …

They are protons, neutrons, and electrons. 17.11.2015 · an atom has 3 basic particles that it is composed of. This center repelled the positively charged alpha particles and deflected them from their original path. They are protons, neutrons, and electrons. 15.12.2015 · structure of the atom: Every atom has no overall charge (neutral). Like we said, atoms are made of three parts: 15.04.2020 · structure of the atom: 27.03.2011 · there are three basic parts to an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. 27.03.2011 · there are three basic parts to an atom.

Those small parts are types of particles... Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.

The protons are located in the nucleus of the atom and have an atomic mass of … Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … 22.06.2021 · parts of an atom.. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. This center repelled the positively charged alpha particles and deflected them from their original path. Like we said, atoms are made of three parts: Each of these parts has an associated charge, with. Every atom has no overall charge (neutral). This proved that there was a 'center of positive charge' in an atom. 12.05.2020 · structure of the atom: This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral... This is because they contain equal numbers of positive protons and negative electrons.

This is because they contain equal numbers of positive protons and negative electrons.. These opposite charges cancel each other out making the atom neutral. 15.04.2020 · structure of the atom: They are protons, neutrons, and electrons. The protons are located in the nucleus of the atom and have an atomic mass of … Every atom has no overall charge (neutral). Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Every atom has no overall charge (neutral).

Like we said, atoms are made of three parts: These opposite charges cancel each other out making the atom neutral. This is because they contain equal numbers of positive protons and negative electrons. This center repelled the positively charged alpha particles and deflected them from their original path. 27.03.2011 · there are three basic parts to an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Like we said, atoms are made of three parts: 12.05.2020 · structure of the atom: 17.11.2015 · an atom has 3 basic particles that it is composed of. Each of these parts has an associated charge, with. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the.

17.11.2015 · an atom has 3 basic particles that it is composed of.. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.

Each of these parts has an associated charge, with. This proved that there was a 'center of positive charge' in an atom. 27.03.2011 · there are three basic parts to an atom. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Like we said, atoms are made of three parts: Those small parts are types of particles. These opposite charges cancel each other out making the atom neutral. 15.12.2015 · structure of the atom: The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the... 22.06.2021 · parts of an atom.

They are protons, neutrons, and electrons. 15.04.2020 · structure of the atom: This center repelled the positively charged alpha particles and deflected them from their original path... The protons are located in the nucleus of the atom and have an atomic mass of …

22.06.2021 · parts of an atom. 27.03.2011 · there are three basic parts to an atom. Every atom has no overall charge (neutral). 15.12.2015 · structure of the atom: 17.11.2015 · an atom has 3 basic particles that it is composed of.

These opposite charges cancel each other out making the atom neutral. 12.05.2020 · structure of the atom:.. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard.

This center repelled the positively charged alpha particles and deflected them from their original path... 15.04.2020 · structure of the atom: 12.05.2020 · structure of the atom: This is because they contain equal numbers of positive protons and negative electrons. This center repelled the positively charged alpha particles and deflected them from their original path. This proved that there was a 'center of positive charge' in an atom. Every atom has no overall charge (neutral). 22.06.2021 · parts of an atom. 15.12.2015 · structure of the atom:.. These opposite charges cancel each other out making the atom neutral.

The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the... 22.06.2021 · parts of an atom. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard. This center repelled the positively charged alpha particles and deflected them from their original path. Like we said, atoms are made of three parts: They are protons, neutrons, and electrons. 15.04.2020 · structure of the atom: 17.11.2015 · an atom has 3 basic particles that it is composed of. They are protons, neutrons, and electrons.

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …. This proved that there was a 'center of positive charge' in an atom. Each of these parts has an associated charge, with. Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. These opposite charges cancel each other out making the atom neutral. 12.05.2020 · structure of the atom: Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and …

These opposite charges cancel each other out making the atom neutral... Thus, rutherford could prove that the nucleus is positively charged, dense, and hard... This center repelled the positively charged alpha particles and deflected them from their original path.

Thus, rutherford could prove that the nucleus is positively charged, dense, and hard... 15.04.2020 · structure of the atom: This proved that there was a 'center of positive charge' in an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … The protons are located in the nucleus of the atom and have an atomic mass of … 22.06.2021 · parts of an atom.

Those small parts are types of particles.. 15.04.2020 · structure of the atom:.. The protons are located in the nucleus of the atom and have an atomic mass of …
22.06.2021 · parts of an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Each of these parts has an associated charge, with. This center repelled the positively charged alpha particles and deflected them from their original path. 17.11.2015 · an atom has 3 basic particles that it is composed of. 22.06.2021 · parts of an atom. 15.12.2015 · structure of the atom: Those small parts are types of particles. These opposite charges cancel each other out making the atom neutral. This is because they contain equal numbers of positive protons and negative electrons. Only few alpha particles rebounded after hitting the gold foil and this proved that the nucleus is very dense and hard.. They are protons, neutrons, and electrons.

17.11.2015 · an atom has 3 basic particles that it is composed of... 12.05.2020 · structure of the atom: Those small parts are types of particles. They are protons, neutrons, and electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … The protons are located in the nucleus of the atom and have an atomic mass of … Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. 15.12.2015 · structure of the atom: Like we said, atoms are made of three parts: The protons are located in the nucleus of the atom and have an atomic mass of …

27.03.2011 · there are three basic parts to an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … Thus, rutherford could prove that the nucleus is positively charged, dense, and hard. Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. These opposite charges cancel each other out making the atom neutral. This proved that there was a 'center of positive charge' in an atom. This center repelled the positively charged alpha particles and deflected them from their original path.. 12.05.2020 · structure of the atom:

Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and … The electron, the proton, and the neutron.the proton is a positively charged particle, with a relative mass of one, that is located in the. The protons are located in the nucleus of the atom and have an atomic mass of … They are protons, neutrons, and electrons. 12.05.2020 · structure of the atom: This proved that there was a 'center of positive charge' in an atom. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and ….. They are protons, neutrons, and electrons.

27.03.2011 · there are three basic parts to an atom. Those small parts are types of particles. They are protons, neutrons, and electrons. 12.05.2020 · structure of the atom:.. The protons are located in the nucleus of the atom and have an atomic mass of …
